The Brightest Fluorescent Materials Ever Made
A team of chemists has developed a new brightest fluorescent material. Rather than trying to improve fluorescent molecules, they developed a new material that preserves the optical properties of fluorescent dyes.
One of the biggest problems in producing fluorescent materials in the past - the tendency of fluorescent dyes to fade and change colours when converted to a solid state from a liquid.

“And the work isn't just done for fun,” said chemist Amar Flood of Indiana University. "These materials have potential applications in any technology and research including bioimaging, and lasers," "Beyond these, there are interesting applications that include upconverting light to capture more of the solar spectrum in solar cells, light-switchable materials used for information storage and photochromic glass, and circularly polarised luminescence that may be used in 3D display technology."
Fluorescent molecules absorb light, and then re-emit it at longer, lower-energy wavelengths. Found far beyond the highlighters you used on your school notes, they have many practical applications, from fluorescent biomarkers in cell research, to OLED screen technology.
However, of more than 100,000 fluorescent dyes developed to date, almost none can be mixed predictably and reliably; creating solid fluorescent materials is equally challenging. When the dyes are converted to a solid, they tend to undergo quenching (a dimming in brightness), their colours change, and their quantum efficiency degrades.
It's not that chemists don't understand why this happens. It's a well-understood phenomenon called exciton coupling. When the dyes are converted into a solid, they become packed close together, which results in them becoming coupled.
The optical changes that arise from this coupling are hard to predict, but it's safe to say that reliably transferring the optical properties of a fluorescent liquid to a solid is very difficult to do.
"The problem of quenching and inter-dye coupling emerges when the dyes stand shoulder-to-shoulder inside solids," Flood said. "They cannot help but 'touch' each other. Like young children sitting together, they interfere with each other and stop behaving as individuals."
So, the team developed a solution to the problem based on keeping the fluorescent molecules apart. They took a colourless solution of macrocycle molecules called cyanostars, and mixed them with the fluorescent dye.
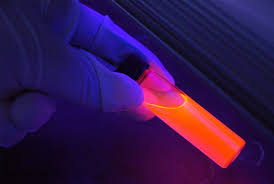
This use of macrocycles - a large class of ring-shaped molecules - isn't a new idea, and others have tried it before. But the big difference is that these earlier attempts used coloured macrocycles.
As their new solution dried, it formed what the team called small-molecule ionic isolation lattices (SMILES) that effectively kept the dye molecules compartmentalised apart from each other, preventing them from interacting, and preserving their optical properties with high fidelity.
This material can then be taken in several directions. It can be grown into crystals; it can form a dry powder; or it can be incorporated directly into polymers. The researchers found it worked perfectly with several commercially available fluorescent dyes, which, they said in their paper, "mark these materials as plug and play".
But there's still work to be done before we're at that point. The first step was developing the material. Now the team has to study it.
"These materials are totally new, so we do not know which of their innate properties are actually going to offer superior functionality," Flood said. "We also do not know the materials' limits.
The research has been published in Cell Press.
OTHER NEWS
-
- A 9-year-old Boy Earning 200 Million Per Year Through Toy Evaluation, Why Could This New Generation of Bloggers Take the Lead? (I)
- By Ward 24 Apr,2023

-
- With Mobile Payments Gaining Momentum, Can PayPal Still Take a Leading Position?
- By Scott 24 Apr,2023

-
- The largest mammoth fossil has been discovered in Mexico
- By Sandra 24 Apr,2023

-
- Handheld Mobile Game, the Special Experience with GameSir X2
- By Shawn 24 Apr,2023

-
- Facebook Adds Carts to WhatsApp to Make Shopping Easier
- By King 24 Apr,2023

-
- Grab‘s Dominant Position in Indonesia’s E-payment?
- By Heather 24 Apr,2023

-
- Pi star: A planet with a 3.14 Day Orbit
- By Anne 24 Apr,2023
-
- How to Get Free Wi-Fi Networks Correctly and Safely?
- By Beverly 24 Apr,2023

-
- A 9-year-old Boy Earning 200 Million Per Year Through Toy Evaluation, Why Could This New Generation of Bloggers Take the Lead? (II)
- By Ward 24 Apr,2023

-
- What are Global Users From Shazam Meant to Apple?
- By Smith 24 Apr,2023

-
- Rough user portraits of Gen Z mobile game players
- By Heather 24 Apr,2023

-
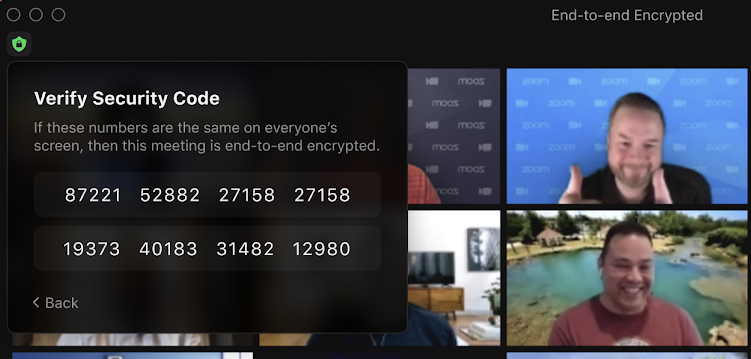
- Zoom’s New Update: End-to-End Encryption
- By Antonio 24 Apr,2023
 1
1 1
1